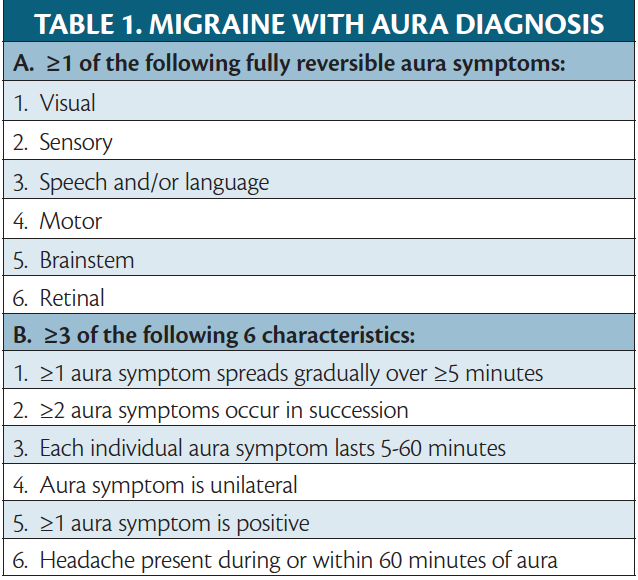
Migraine is a neurobiologic disease in which a genetic predisposition combined with environmental influences leads to neurologic dysfunction, of which headache is the most common symptom. Migraine is defined as a headache, typically unilateral with a pulsating quality and moderate-to-severe pain intensity aggravated by or causing avoidance of routine physical activity and lasting 4 to 72 hours in adults and 2 to 72 hours in children. Only 2 of the 4 listed symptoms are needed for diagnosis.1 The headache is accompanied by nausea/vomiting with photophobia/phonophobia.1 Migraine is associated with an aura in about 30% of people with the disease,2 and is the onset of fully reversible symptoms that progress slowly before or during the headache. Aura is considered 1 of the 4 phases of a migraine attack (Table 1).3
Those with first-degree relatives who have migraine with aura have a higher risk of developing the disease compared with those whose first-degree relatives have migraine without aura.4 No specific gene associated with migraine with aura has been identified, except in familial hemiplegic migraine, a largely monogenic disorder.
The most common type of aura is visual (see Migraine Visual Aura & Other Visual Phenomena in this issue); however, other types of aura symptoms can be seen with migraine instead of or in addition to visual aura. Nonvisual auras comprise sensory, motor, and language symptoms. Considering aura variability among people with migraine and even between migraine attacks in an individual with migraine, there is a focus on how auras are influenced by migraine pathophysiology and how they could be managed. Better understanding of different types of migraine aura will help providers appropriately diagnose migraine aura, and better understanding of the pathophysiology will allow providers to give safe and effective treatment as well as education to people who experience what can be frightening symptoms. This review discusses the different types of nonvisual aura associated with migraine.
Pathophysiology of Aura
The aura phase of a migraine attack is thought to be due to cortical spreading depression (CSD), which is a slowly propagating wave of depolarization followed by cortical inhibition for up to 30 minutes and then depression of or decrease in electrical activity.5 The wave of CSD causes physiologic changes of hyperemia followed by a prolonged phase of oligemia.6,7 These changes are due to electrolyte and neurotransmitter fluctuations that propagate from the nerve to adjacent cells.3 Thus, blood vessel changes result from CSD and not the direct cause of aura. Because these changes are due to electrophysiologic changes rather than decreased blood flow, aura symptoms are reversible. CSD also explains why aura symptoms occur progressively with slow spread before resolving, whereas vascular symptoms are immediate and sometimes irreversible. Hypoperfusion that occurs with aura does not reach the level of ischemic changes.
CSD has been seen on advanced neuroimaging (ie, functional MRI [fMRI]) in those with visual aura as functional connectivity differences in the extrastriatal cortex.8 Perfusion changes on fMRI start in the occipital cortex and spread anteriorly with progression of visual aura.6 Notably, individuals with nonvisual aura have higher resting state fMRI differences in the left lingual gyrus (within the visual network) and the right anterior insula (within the sensorimotor network) compared with people who have migraine with simple visual aura or without aura. These differences suggest different aura semiologies may originate in different parts of the brain.9
Most people with migraine do not experience aura, and those who do may not experience aura in all their attacks. Auras may also occur without migraine headache. Whether CSD triggers migraine attacks, is a result of the true inciting mechanism, or happens independently remains unknown. More research is needed to better understand aura in relation to other phases of the migraine attack, particularly the prodrome that is initiated in other parts of the brain.3,5
Sensory Aura
Sensory symptoms are the second most common migraine aura according to a prospective diary study,10 and have been described as anesthesia/hypoesthesia (ie, decreased or no sensation) or more commonly as paresthesias (ie, heightened sensation or a pins-and-needles feeling). Sensory auras are typically unilateral and most commonly occur on the face, periorbital area, or hand.10 Like visual auras, sensory aura progresses from onset and sometimes travels from 1 area to another before fully resolving. Sensory auras may be unilateral or contralateral if the headache is unilateral. The thalamus is thought to play a role in the positive symptoms of paresthesia. Loss of excitatory input to GABAergic nuclei leads to increased sensory excitation.11 Migraine with unilateral motor symptoms (MUMS), discussed later, has been noted in clinical practice to be associated with sensory aura and allodynia.12
Language Aura/Dysphasic Aura
Dysphasic auras are difficulties with language and are the third most common aura in a prospective diary study. Symptoms most commonly include paraphasic errors (ie, word substitution) and word-finding difficulty.10 These are considered specific language difficulties, which are time limited (5-60 min), in contrast to cognitive slowing, or brain fog, that occurs during the prodrome and may lead a person to feel they are struggling with language. Prodrome symptoms, which occur hours to days before migraine headache onset, are much more common and relate to decreased concentration rather than CSD.
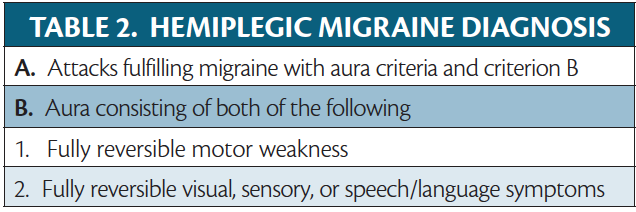
Hemiplegic Migraine
In hemiplegic migraine, aura involves unilateral motor weakness (Table 2). Hemiplegic migraine is divided into the subtypes familial hemiplegic migraine (FHM) and sporadic hemiplegic migraine (SHM). FHM runs in a family with at least 1 first- or second-degree relative who has hemiplegic migraine. SHM occurs pontaneously without family history. In FHM there is typically an identifiable genetic variant. Symptoms of hemiplegic migraine often mimic stroke. Considering the rarity of hemiplegic migraine, it is important to rule out the more common cerebrovascular disease.
Like other auras, hemiplegic aura typically occurs unilaterally and spreads throughout the body, often starting in the hand and spreading up to the arm and face. The upper extremities are affected more often than the lower extremities. Persons with FMH1 or FHM2 also may have cerebellar signs. During an attack, neurologic exam findings of central nervous system dysfunction (eg, hyperreflexia and upgoing plantar reflex) may be observed. The aura lasts 20 to 30 minutes and sometimes up to an hour and occurs before or during a headache typical of migraine.13 Some with prolonged aura can have weakness that lasts for weeks.14 Hemiplegic migraine is differentiated from stroke by the progression of neurologic dysfunction followed by a headache, and no matter how long the aura lasts, it is completely reversible.
FHM
FHM is further divided into 4 subtypes, typically related to the gene in which a variant is identified. FHM1 is associated with variants in the α-1A subunit of the P/Q-type Cav2 voltage-gated calcium channel (CACNA1a), also associated with cerebellar degeneration and epilepsy. FHM2 is associated with variations in the α2 subunit of sodium-potassium ATPase (ATP1A2). FHM3 is associated with variants in the sodium voltage-gated channel α subunit 1 (SCN1A), a cerebral sodium channel. In FHM4, affecting 25% of those with FHM, no gene variant is identified.14 Genetic variants in FHM types 1, 2, and 3 affect ion channels and electrolyte transport involved in neuronal excitation and inhibition and are thought to decrease the threshold for CSD. Persons with hemiplegic migraine are more likely to have auras that are more likely to be more severe.
MUMS
Although hemiplegic migraine is rare, presentations of weakness associated with migraine attacks are not. MUMS is an aura syndrome that is phenotypically similar to, but believed distinct from, hemiplegic migraine, although it is not yet included in the International Classification of Headache DIsorders (ICHD).12 MUMS presents as ipsilateral weakness that spreads in a rostrocaudal (ie, nose to tail) direction and is associated with concurrent sensory complaints (eg, allodynia or paresthesia). On exam, persons with MUMS have a sudden loss of resistance during muscle strength testing (termed give-way weakness) rather than true weakness, which is not the case in hemiplegic migraine. The weakness in MUMS is thought to have a different etiology than hemiplegic migraine, related to the pain and presence of allodynia. In some cases, this entity can fall into the category of functional neurologic disease, whereas in others, it is directly from pain, and in others may simply be a subjective weakness due to proprioceptive loss or a sensation of heaviness. Considering the slight difference in examination between the 2 entities, however, it can be difficult to differentiate between MUMS and hemiplegic migraine. It is important to distinguish between MUMS and hemiplegic migraine because it affects whether genetic testing is needed and may affect the therapeutic plan.
Treatment
Rarity of the condition and lack of randomized clinical trials make treatment of hemiplegic migraine challenging. For the most part, treatment is similar to what is used for migraine with and without aura—a combination of preventive therapies, acute treatments, and lifestyle changes. Because there are concerns for the vasoactive properties of ergotamines and triptans in hemiplegic migraine, traditionally these were thought to be contraindicated. Retrospective review, however, has shown triptans to be safe and without an increase in stroke risk when used for hemiplegic migraine.15 Considering the new gepant class of migraine medications do not have vasoconstrictive properties, many practitioners are opting for this treatment instead. Treating the aura of hemiplegic migraine has been focused on affecting CSD because there is thought to be a lower threshold for CSD in hemiplegic migraine. Specific targets include NMDA and glutamate channel, which can be targeted with intranasal ketamine for acute treatment.16 For preventive measures, case reports and series have suggested treatments such as furosemide, magnesium, verapamil, and acetazolamide are effective.14
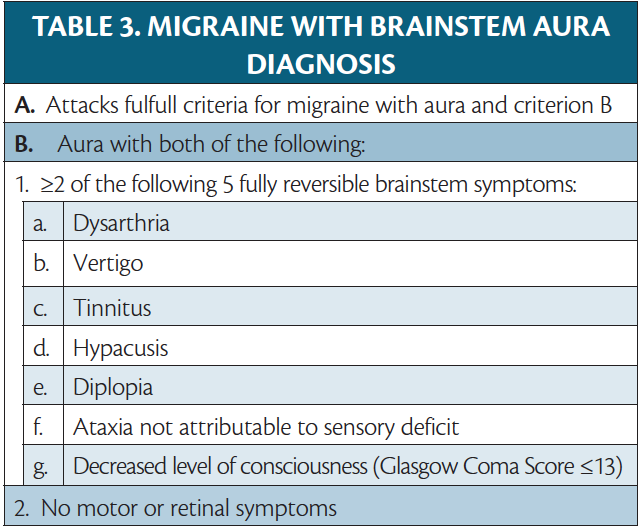
Brainstem Aura
Migraine with brainstem aura (MWBA) is defined as migraine with aura that includes at least 2 of the following symptoms, suggesting brainstem involvement: dysarthria, vertigo, tinnitus, hyperacusis, diplopia, ataxia, and decreased level of consciousness (Table 3).1 The symptoms are reversible, and multiple symptoms may occur in sequence, often beginning with a visual aura. Vertigo is the most common symptom, occurring in up to 63% of persons with MWBA.17 If motor symptoms are present, the diagnosis of hemiplegic migraine should be made instead. MWBA was first termed basilar artery migraine in a report of 2 cases with identical symptoms attributed to abnormalities of the basilar artery circulation.18 These cases were in a person, age 14 years, who had a 15-minute aura followed by a severe headache and vomiting, and a person, age 65, who ultimately became comatose and died due to a basilar artery thrombosis. It was assumed that these unusual aura symptoms were the result of basilar artery constriction, and those with basilar migraine (ie, MWBA) have long been counseled to avoid vasoconstrictors (eg, triptans and dihydroergotamine [DHE]), despite a lack of evidence for this assumption.15 As no evidence for vasospasm as the cause of migraine has been found, the term basilar artery migraine has been replaced with MWBA.19
The exact pathophysiology of MWBA is still not well understood. Some have suggested these aura symptoms may have cortical origins and can be attributed to CSD, which may explain why visual aura often accompanies MWBA symptoms.17 MWBA symptoms are also common in hemiplegic migraine attacks, which are of cortical origin. A study suggests that aura with true brainstem origin does exist, but is rare and not well captured by the current ICHD criteria.19 The authors argue that in patients with multiple aura symptoms that would involve widespread areas of the cortex, including bilateral visual and sensory symptoms, aura is better explained by brainstem dysfunction than CSD. Using stricter criteria requiring face-to-face evaluation by a neurologist, MRI, and at least 3 reversible brainstem symptoms, they found only 1% to 2% of cases of migraine with aura were convincingly MWBA.19
The most important clinical question regarding MWBA is whether vasoconstrictors (eg, triptans and DHE) should be used or are contraindicated in MWBA, which has kept those with MWBA typically excluded from clinical trials. Although a retrospective review of persons with MWBA who used triptans or DHE did not show an increased risk of stroke or myocardial infarction,15 these medications still list basilar migraine as a contraindication, leading to underutilization for MWBA. As more acute migraine treatments have become available, it is certainly reasonable to try nonvasoconstrictive medications first, but there is no compelling evidence to support withholding these effective medications from people with MWBA.
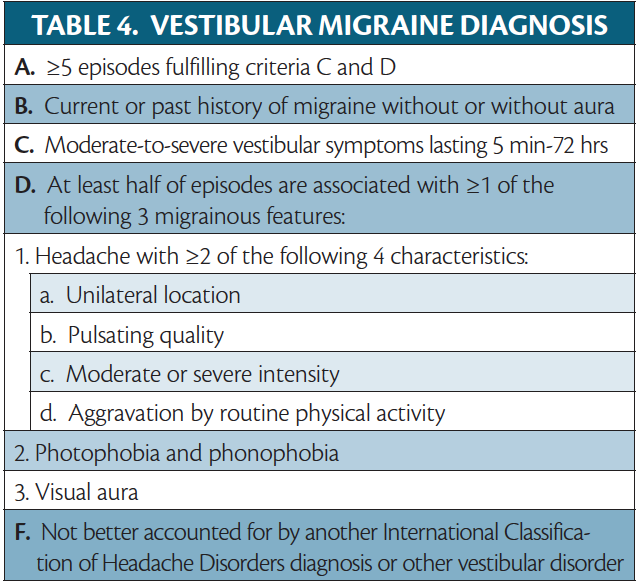
Vestibular Migraine
Vestibular migraine (VM) is not a form of aura but discussed to differentiate it from MWBA (Table 4). VM is defined as vestibular symptoms in a person with migraine, with at least half of attacks having migrainous features (ie, migraine headache, photophobia/phonophobia, and/or visual aura).1 Vestibular symptoms of VM are internal or external spontaneous vertigo; positional vertigo, which occurs after a change in head position; visually induced vertigo, which is triggered by a complex or moving visual stimulus; head motion-induced vertigo, which occurs during head motion; and head motion-induced dizziness with nausea. Internal vertigo is a false sense of self-motion, and external vertigo is a false sensation that the visual surroundings are spinning or flowing. Dizziness is a sensation of disturbed spatial orientation.1 Other commonly reported symptoms include vision changes (eg, blurriness, oscillopsia, and visual lag , which is a sensation of the visual surround lagging behind head movement) as well as cognitive symptoms (eg, disorientation, cognitive slowing, fatigue, and word-finding difficulty.20 VM may also present with auditory symptoms (eg, tinnitus and hearing changes).20
The constellation of vertigo, tinnitus, and hypacusis may lead to an incorrect diagnosis of MWBA, but a careful history of the timeline and order of symptoms should enable a clinician to distinguish between these diagnoses. The duration of vestibular symptoms in VM ranges from minutes to days, and symptoms may come and go throughout a migraine attack, in contrast to the 5 to 60 minutes of aura just before or with the onset of a headache typical of a migraine aura.1 Persons with VM may also experience less severe symptoms interictally, whereas aura symptoms should fully resolve.20 There can be considerable variability in the symptoms and duration of VM attacks not usually seen in aura.21 VM is a far more common cause of dizziness or vertigo in migraine, affecting up to 3% of the general population, making it the most common neurologic cause of vertigo.20 Dizziness is the most common nonheadache symptom reported by people with migraine, and at least one-third of people with migraine experience vertigo during their lifetime.21 Vertigo is the most common symptom in MWBA, but MWBA itself is quite rare in comparison.17
Conclusion
Migraine auras are fascinating phenomena that may present with many unusual neurologic symptoms that can be alarming to patient and clinician alike. Once identified, aura can often be well managed with migraine treatments. Studying migraine auras has deepened our understanding of migraine pathophysiology, and further research into less common forms of aura may further expand our knowledge of this disabling disease.
1. International Headache Society. The International Classification of Headache Disorders, 3rd edition. Accessed March 11, 2022. https://ichd-3.org
2. Rasmussen BK, Olesen J. Migraine with aura and migraine without aura: an epidemiological study. Cephalalgia. 1992;12(4):221-228.
3. Dodick DW. A phase-by-phase review of migraine pathophysiology. Headache. 2018;58(Suppl 1):4-16.
4. Russell MB, Iselius L, Olesen J. Migraine without aura and migraine with aura are inherited disorders. Cephalalgia. 1996;16(5):305-309.
5. Lai J, Dilli E. Migraine Aura: updates in pathophysiology and management. Curr Neurol Neurosci Rep. 2020;20(6):17.
6. Hadjikhani N, Sanchez Del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98(8):4687-4692.
7. Olesen J, Larsen B, Lauritzen M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann Neurol. 1981;9(4):344-352.
8. Russo A, Silvestro M, Tessitore A, Tedeschi G. Shedding light on migraine with aura: the clarifying role of advanced neuroimaging investigations. Expert Rev Neurother. 2019;19(8):739-750.
9. Silvestro M, Tessitore A, Di Nardo F, et al. Functional connectivity changes in complex migraine aura: beyond the visual network. Eur J Neurol. 2022;29(1):295-304.
10. Viana M, Sances G, Linde M, et al. Clinical features of migraine aura: results from a prospective diary-aided study. Cephalalgia. 2017;37(10):979-989.
11. Bolay H, Vuralli D, Goadsby PJ. Aura and head pain: relationship and gaps in the translational models. J Headache Pain. 2019;20(1):94.
12. Young WB, Gangal KS, Aponte RJ, Kaiser RS. Migraine with unilateral motor symptoms: a case-control study. J Neurol Neurosurg Psychiatry. Jun 2007;78(6):600-604.
13. Kumar A, Samanta D, Emmady PD, Arora R. Hemiplegic migraine. In: StatPearls. StatPearls Publishing; 2022. Updated February 4, 2022. Accessed March 18, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513302/
14. Di Stefano V, Rispoli MG, Pellegrino N, et al. Diagnostic and therapeutic aspects of hemiplegic migraine. J Neurol Neurosurg Psychiatry. 2020;91(7):764-771.
15. Mathew PG, Krel R, Buddhdev B, et al. A retrospective analysis of triptan and DHE use for basilar and hemiplegic migraine. Headache. 2016;56(5):841-848.
16. Kaube H, Herzog J, Kaufer T, Dichgans M, Diener HC. Aura in some patients with familial hemiplegic migraine can be stopped by intranasal ketamine. Neurology. 2000;55(1):139-41.
17. Demarquay G, Ducros A, Montavont A, Mauguiere F. Migraine with brainstem aura: why not a cortical origin? Cephalalgia. 2018;38(10):1687-1695.
18. Bickerstaff ER. The basilar artery and the migraine-epilepsy syndrome. Proc R Soc Med. 1962;55:167-169.
19. Yamani N, Chalmer MA, Olesen J. Migraine with brainstem aura: defining the core syndrome. Brain. 2019;142(12):3868-3875.
20. Beh SC, Masrour S, Smith SV, Friedman, DI. The spectrum of vestibular migraine: clinical features, triggers, and examination findings. Headache. 2019;59(5):727-740.
21. Baloh RW. Vestibular migraine I: mechanisms, diagnosis, and clinical features. Semin Neurol. 2020;40(1):76-82.
CIW and CC report no disclosures